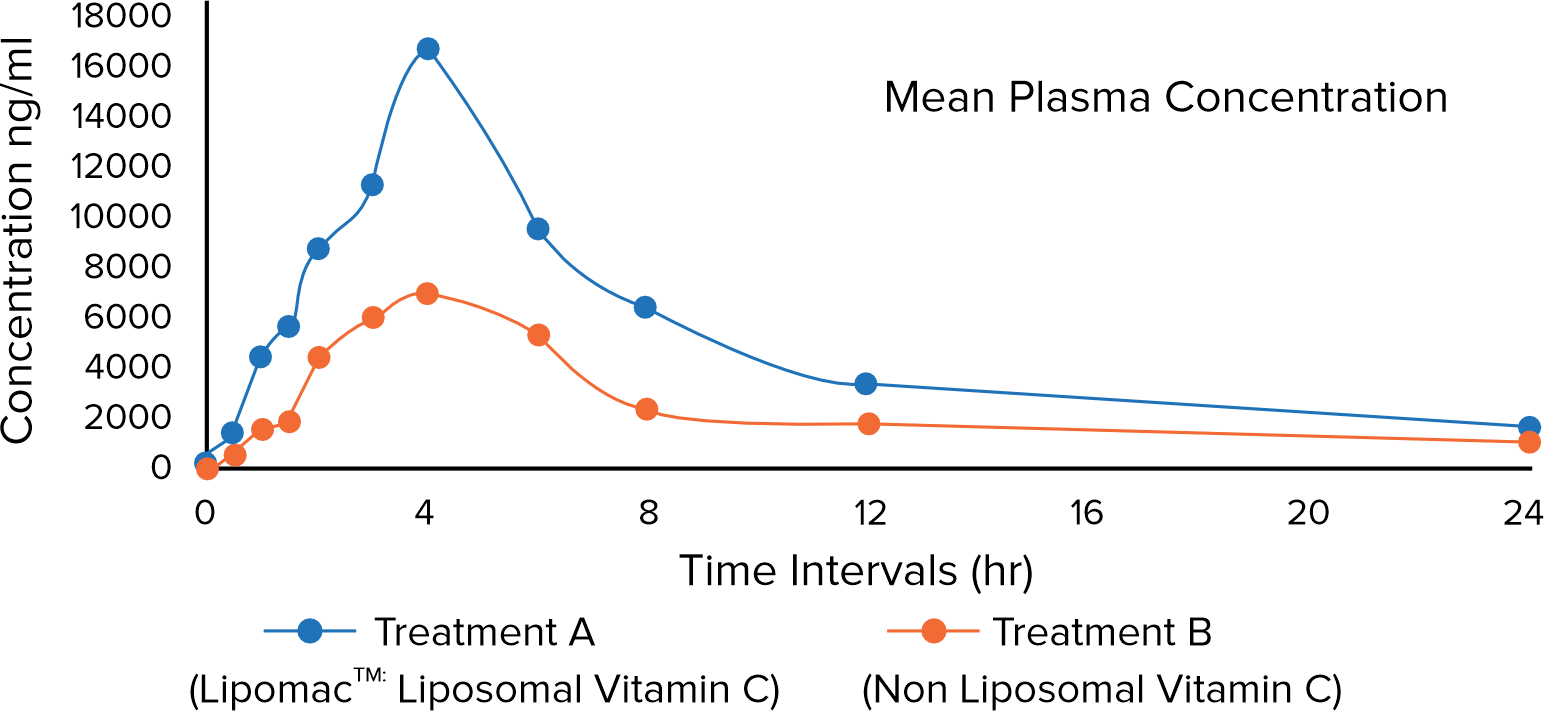
Evaluating the Clinical Impact of Lipomac™: Insights into Bioavailability
Objective: To compare the bioavailability of Lipomac™ —our Liposomal Vitamin C formulation—against a non-liposomal Vitamin C supplement in healthy adult volunteers.
Study Details:- Participants: 12 healthy adults
- Test Product: Lipomac™ (Liposomal Vitamin C) – 500mg (2 capsules per day)
- Reference Product: Non-Liposomal Vitamin C – 500mg (2 capsules per day)
- Administration: Oral intake of 500mg (2 capsules per day)
Results: The study assessed the safety and pharmacokinetic parameters to determine the bioavailability of Lipomac™. Findings revealed that Lipomac™ offers significantly enhanced bioavailability compared to the non-liposomal Vitamin C. Specifically, Lipomac™ demonstrated superior absorption, with bioavailability approximately 2.36 times higher than that of the non-liposomal Vitamin C.