Formulation and characterization of Liposomal glutathione as a key component in mitigation of Kwashiorkor disease in children with malnutrition
Shivaprasad HN, Arnab Chatterjee, Gaurav Soni, Madhu Krishnamani, Sravani T, Preethi PakalaBotanic Healthcare Pvt. Ltd., TSIIC Industrial Development Area, Hyderabada, India
Abstract
- Background. Kwashiorkor disease is due to deficiency of protein in malnutrition condition. Malnutrition is one of the major drawbacks among the Indian children. In India, the impact of undernutrition among impoverished children is disproportionately felt in rural areas. Due to the unequal distribution of food within the household and the consumption of foods with low nutritional qualities, increases the percent of malnutrition in children.
- Objective. To formulate liposomal glutathione as a potent high antioxidant agent for delivery nutritional effect in nutraceutical formulations and other food supplements for boosting immunity through combating Kwashiorkor disease during malnutrition condition in children.
- Method. Nanoliposome of glutathione was formulated by ethanol injection method via formation of organic and aqueous phases. Further, morphology of the formulated liposomal glutathione was characterized using cryo-TEM and confirmed for suitability in nutraceuticals as immune enhancers in children.
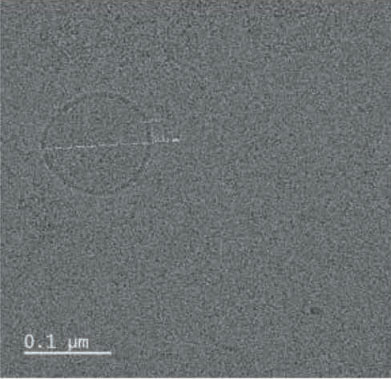
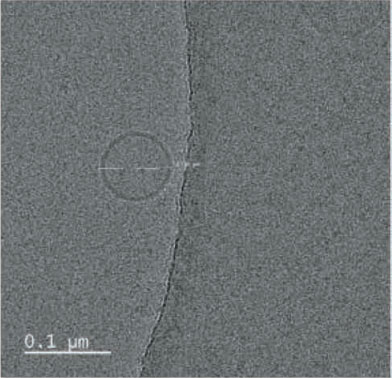
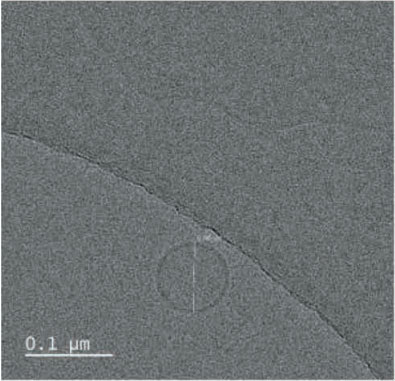
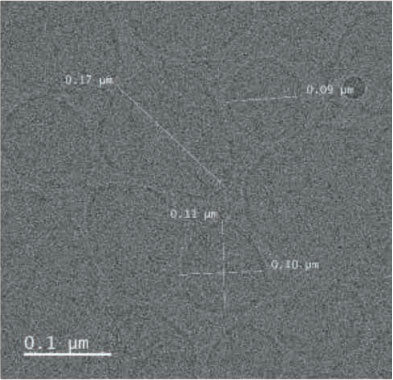
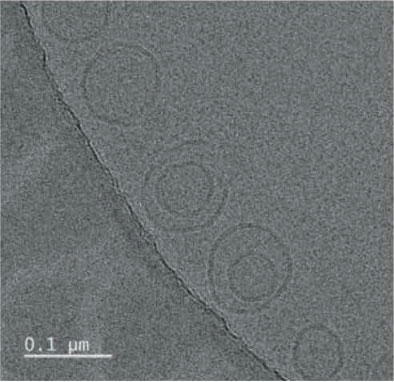
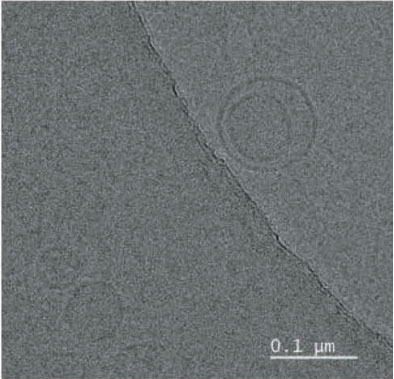
- Result. Glutathione was successfully entrapped in lipid as nanoformulations. Cyro-TEM analysis resulted the shape of the particles are spherical with 0.1 µM average diameter.
- Conclusion. The overall result concluded that liposomal glutathione is very effective in formulation of nutraceutical as well as food supplements as potent immune booster for children and reduce protein deficiency in malnutrition to cure Kwashiorkor disease.
Keywords: Cryo-TEM, glutathione, nanofoumulation, nutraceuticals
Introduction
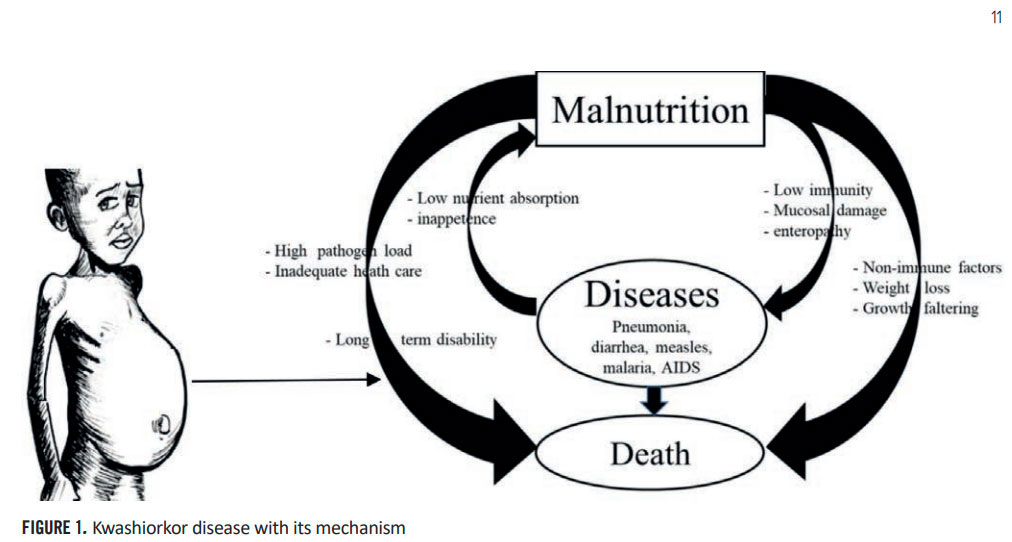
Malnutrition in children is one of the main causes of poverty. Especially in low economic countries are facing such situation among the people especially among the children [1]. Kwashiorkor is a protein deficiency illness brought on by a protracted period of low-quality or inadequate protein consumption [2]. Hence, Protein deficiency is having direct relation with the malnutrition, and Kwashiorkor is one of the very common diseases in children (Figure 1). The mechanism for the same was depicted in figure 1 where the relation of Kwashiorkor disease with the malnutrition has described [3]. Child malnutrition is a persistent issue and ongoing difficulty in India like countries. It was reported that in India, children below 5 years age, death rate is 69 per cent with malnutrition in the year 2022 [4]. Indian Government as well as WHO have taken various standard treatment using F-100 [5]. Apart from, various food supplements are also marketed vastly as nutraceutical products in mitigation of malnutrition in children. There are many ingredients are used for nutraceutical formulation as food supplement. Among them, liposomes are crucial for enhancing the bioavailability, transport, and stability of bioactive substances in nutraceuticals and functional food systems. The unique bilayer hydrophilic and lipophilic structure of these liposomes acquire great advantages like higher encapsulation efficiency, solubility of Bioactive compounds, and delivery of various substances, including nutrients, and drugs, to specific targets in the body [6]. The bioactive compounds of various nutraceuticals and botanical extracts lack water solubility, poor stability, and have fast metabolism leading to poor oral bioavailability. Nutraceuticals often act as both preventive and curative measures, containing vitamins, minerals, antioxidants, probiotics, amino acids, and other bioactive compounds that are isolated or concentrated to provide both nutritional and health benefits [7]. In the nutraceuticals industry, liposomes are employed to enhance bioavailability and absorption by encapsulating these compounds within liposomes, their stability can be improved, and they can be protected from degradation in the digestive system, leading to more effective delivery and utilization in the body. The microencapsulation technology provides a protective barrier around bioactive compounds, increases bioavailability, product solubility, increases resistance to acids and digestive enzymes. It allows for more precise targeting of the encapsulated ingredient in the preparation of food and nutraceuticals. Glutathione is a naturally occurring tripeptide antioxidant present in various fluids, tissues, and cells. Glutathione is an amino acid composed of cysteine, glycine, and glutamic acid [8]. It is vital for reducing oxidative stress, boosting metabolic detoxification, preserving redox equilibrium, and monitoring the immune system. It is crucial to maintain glutathione levels in the prevention of oxidative stress-related diseases. Studies are concentrated on liposome-mediated targeted administration because glutathione is susceptible to degradation in the acid environment of the stomach. As a secure substitute for maintaining neuronal glutathione, investigations on liposomal formulations of glutathione enclosed in lipids developed from lecithin and glycerol. Recent research has demonstrated the antioxidant and anti-atherogenic properties of this encapsulation technology for mass production of nutraceuticals and to improve healthy life among the children [9].
Materials And Method
Materials requires: Glutathione 98%, soy lecithin, ethanol, homogenizer, spray dryer, metal detector and sieve. Numerous techniques are available to formulate liposomes. Botanic Healthcare uses the ethanol injection method in production of liposomal glutathione of 100 grams. Preparation of Liposomal Glutathione involves two phase organic and aqueous phases. Organic phase includes preparation of Lipid solution by mixing ethanol 3:2 grams with soy phosphatidylcholine 70% of 3.5 grams and glycerol of 4 grams and then was dissolved all the ingredients at rate of 8000-10000 rpm under sonication tillhomogenous phase at 55-60°C to create a lipid mixture. Dissolve the sourced glutathione powder of 55 grams in distilled water at an ambient temperature to create glutathione solution. Then, gradually inject the above prepared lipid mixture into the glutathione solution and ensure proper and steady synthesis of liposomes followed by high sheer homogenization of 1000-2000 bar for 3-4 cycles and high-pressure homogenization to further improve liposome formation by reducing particle size. Followed by spray drying, metal detection and sterilization, sieving and storage of liposomal L-Glutathione sample BHC12335KR.
Analysis
TEM sample preparation and AnalysisAround 1 % solution of water/ Iso propyl alcohol (IPA) diluted suspension was prepared and sonicated for 30 minutes. Briefly a drop of the above suspension was place on a copper grid and the filter paper was used to eliminate the excess. After adding a drop of 2% (w/v) uranyl acetate aqueous solution, the sample was exposed to it for five minutes. Later the grid was washed with water to remove the surplus and kept for drying at room temperature for not less than 2 hours. The morphology of liposomes is studied by cryo-TEM [10]. The liposomal L-Glutathione sample BHC12335KR was analyzed at advanced center for cryo-electron microscopy facility, Indian Institute of Science, Bangalore using the Talos Arctica at 200kV using K2 DED. A TEM picture of the vesicles was obtained with different magnifications.
Result And Discussion
TEM was performed for the formulated liposomal L-Glutathione and result showed in Figure 2. The primary antioxidant in the human body is glutathione, which is a tripeptide composed of cysteine, glycine, and glutamic acids. It comes in two forms: oxidized and reduced. The reduced form of glutathione is the active form and a powerful antioxidant, but its absorption is quite low because stomach acids deplete it. Thus, the best option is to use alternate forms, like liposomal glutathione. Up to 80% more absorption is achieved [11]. The employment of liposomes for medication encapsulation is guided by required criteria obtained from liposome visualization data obtained through microscopic techniques
Transmission Electron Microscopy is a crucial technique for determining the size, shape, and composition of nanoparticles since it allows for the direct visualization of individual particles and even their internal structure. The most effective way to see liposomes in their natural state is using cryoelectron microscopy, in which thin suspension films are vitrified (imaged directly in the electron microscope at liquid nitrogen temperature) by freezing them in place. Negative staining TEM is a faster, easier, and less sophisticated way of imaging liposomes than cryo-EM, yet it is also prone to artifacts. When it comes to TEM, we can understand the particle size and shape, where with cryo-TEM along with the above lamellarity can also be evaluated. Drying and staining were the most often utilized TEM sample preparation methods for liposomes.
In comparison to staining, cryo-TEM or cryogenic Thin aqueous hydrated films that have been vitrified in liquid ethane are utilized to create TEM images before imaging [12]. This method is useful for figuring out the size, shape, internal structure, and lamellarity of liposomes since it permits the examination of liposomes in their most natural state. Rapidly freezing liposome samples has the main benefit of reducing ice crystal formation and protecting proteins or other components. The best type of microscopy for studying liposomes right now is cryoTEM since it doesn’t require chemical fixation, dehydration, cutting, or staining [13]. The shape of vesicles can be impacted by all of these techniques. It is cooled rapidly (typically by submerging the sample in liquid ethane that has reached its melting point) and vitrified in a thin layer of solvent before being photographed at extremely low temperatures, which prevents the medium from changing phase or evaporating in the high vacuum. Applying and then blotting a drop of material onto a carbon-coated grid creates the thin layer. The same way prepared liposomal glutathione produced the similar characterization.
The formulation is applied in various marketed formulation as food supplements and nutraceutical products which is safe and healthy for children (Figure 3).
Conclusion
Glutathione as such is not favorable in acidic stomach pH and hence nanoformultion of the same enhance the bioavailability and absorption in the nutraceutical formulations. In the present investigation, liposomal Glutathione was prepared in laboratory condition using ethanol injection and tested as one of the ingredients for many marketed nutraceutical products. It has potent antioxidant activity and very useful in enhancement of immunity by combating malnutrition in children and curing Kwashiorkor disease by improving the protein content as a main ingredients in ample number of food supplement products in market.
Acknowledgement
Authors are thankful to the Botanic Healthcare Pvt Ltd for provided facilities for perform the formulation and develop various nutraceutical products.
References
- Siddiqui F, Salam RA, Lassi ZS, Das JK. The Intertwined Relationship Between Malnutrition and Poverty. Front Public Health. 2020; 8:453. doi: 10.3389/fpubh.2020.00453
- Sullivan J, Ndekha M, Maker D, Hotz C, Manary MJ. The quality of the diet in Malawian children with kwashiorkor and marasmus. Matern Child Nutr. 2006; 2(2):114-22.
- Michael H, Amimo JO, Rajashekara G, Saif LJ and Vlasova AN. Mechanisms of KwashiorkorAssociated Immune Suppression: Insights From Human, Mouse, and Pig Studies. Front. Immunol. 2022; 13:826268. doi: 10.3389/fimmu.2022.826268.
- Govender I, Rangiah S, Kaswa R, Nzaumvila D. Malnutrition in children under the age of 5 years in a primary health care setting. S Afr Fam Pract (2004). 2021 Sep 7;63(1):e1-e6. doi: 10.4102/safp.v63i1.5337
- Ulahannan SK, Wilson A, Chhetri D, Soman B, Prashanth NS. Alarming level of severe acute malnutrition in Indian districts. BMJ Glob Health. 2022 Apr;7(4):e007798. doi: 10.1136/bmjgh-2021-007798
- Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022 May 13;8(5):e09394. doi: 10.1016/j.heliyon.2022.e09394
- Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med. 2014 Dec;5(12):1487-99.
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009 Feb-Apr;30(1-2):1-12. doi: 10.1016/j.mam.2008.08.006
- Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K, Dheer D, Sharma A, Sangnim T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients. 2022 Nov 3;14(21):4637. doi: 10.3390/nu14214637
- Meister A, Blume A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers. 2017; 9(10):521. https://doi. org/10.3390/polym9100521
- . Sinha R, Sinha I, Calcagnotto A, Trushin N, Haley JS, Schell TD, Richie JP Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur J Clin Nutr. 2018 Jan;72(1):105-11. doi: 10.1038/ejcn.2017.132
- Newcomb CJ, Moyer TJ, Lee SS, Stupp SI. Advances in cryogenic transmission electron microscopy for the characterization of dynamic self-assembling nanostructures. Curr Opin Colloid Interface Sci. 2012 Dec;17(6):350-9. doi: 10.1016/j.cocis.2012.09.004
- Baxa U. Imaging of Liposomes by Transmission Electron Microscopy. Methods Mol Biol. 2018;1682:73-88. doi: 10.1007/978-1-4939-7352- 1_8